Technical Development - Completed December 2012
Robotic Exploration and Sampling of Mars
Final Summary Paper
John Eiler (California Institute of Technology)
Campus PI

Joel Hurowitz (Jet Propulsion Laboratory)
JPL PI
Overview
The technical development program focused on Robotic Exploration and Sampling of Mars ('KISS-Mars'), and its parallel JPL-run program ('New Directions in Robotic Exploration of Mars') received initial funding in October of 2009 and was completed in 2012.
Funding of these programs focused on research concerned with technical capabilities and strategies for in situ (i.e., lander and/or rover) measurements of the ages of Martian rocks, soils and ices. In particular, we focused on methods of K-Ar dating, which is based on the accumulation of 40Ar in K-bearing solids as a result of decay of the naturally occurring, long-lived radioisotope, 40K. Our first year of activity involved initiation of several separate ‘tracks’ toward technical solution of the problems associated with sample handling, Ar extraction and analysis, and K analysis. Several of these methods were, in some sense, in competition with one another, as we assumed we would narrow our focus as some experiments encountered more success than others.
Our approach evolved and focused to the point that we believe publically presented an integrated instrument package for remote dating of planetary materials, with pilot study data showing its accuracy and precision for relatively challenging cases. The following developments have were key to our progress:
Isotope dilution concept: The critical trade-off that one faces in designing a geochronology experiment is between accuracy and precision on one hand, vs. spatial scale and/or sample size on the other. Our initial efforts included several experiments that took one or the other approach. In the last year of this project, we focused our attention on one experiment that makes use of the strategy of ‘isotope dilution’, whereby a sample is physically mixed with a known quantity of a synthetic isotope of an element of interest, and then measured for the abundance ratio of a natural isotope to the synthetic isotope you added. The beauty of this approach is that it automatically corrects for a wide range of analytical artifacts, such as variations in ion efficiency in a mass spectrometer source or matrix (i.e., sample-type) dependent artifacts. Importantly, it can be applied equally to a wide range of materials (basalt, sulfates, carbonates, clays, etc.). The costs of this approach are that one loses any information contained in the spatial heterogeneity of isotopes in the sample (i.e., because it must be intimately mixed with the synthetic isotope, or ‘spike’, generally by fluxed melting), and sensitivity may be reduced if samples are mixed with spike by melting in a flux.
The Farley research group designed an isotope dilution experiment that could be plausibly scaled to a flight instrument, based on the idea that samples (rock chips, cores or powders) are placed in a crucible filled with a flux and isotopic spikes for both Ar and K. The crucible is heated, melting the sample and releasing both the natural and spike Ar isotopes by electron impact mass spectrometry. The crucible is then cooled, leaving a glass, which is measured for its K isotope ratio, by any of a number of different methods (laser ablation, using the JPL in situ mass spectrometry experiment, is shown below).
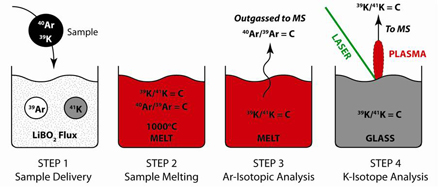
• Step 1: Deliver sample to crucible containing flux and solid-phase spikes
• Step 2: Flux-assisted melting of sample (sample-spike homogenization)
• Step 3: Measure Ar-isotopic composition of released gases
• Step 4: Measure K-isotopic composition of quench glass
• Step 5: Calculate age from 40K/40Ar abundance ratio
Production of spiked fluxes: We identified and characterized LiBO2 fluxes appropriate for melting silicate and oxide samples (we suspect it will also be suitable for sulfates, clays, etc.), and produced or purchased suitable Ar and K isotope spikes.
Study of fluxed melting: We conducted a series of experiments on the melting properties of mixtures of LiBO2 flux with feldspar and basalt samples. These experiments establish that demonstration of the melting properties and homogeneity of fluxed samples can be fused to a melt and then quenched to a relatively homogeneous glass (i.e., even in cation chemistry) over a range of temperatures that should be easily achievable on a flight instrument package.
Ar isotope analysis: The Farley group conducted a series of experiments in which feldspar and basalt samples were fused in isotopically ‘spiked’ flux under vacuum, and the released gas then analyzed by quadrupole mass spectrometer. With appropriate pre-heating of the furnace, this can be done with low background and yields a measurement of sample argon having an accuracy of ±3 % (1s), for samples of young (~175 Ma) terrestrial basalt. This experiment exceeded our original target precision for age determination (~±10 %), on a sample containing an amount of Ar similar to the most Ar-poor targets we might imagine measuring on the surface of Mars or another body.
K analysis: The developments summarized above brought us one step away from a full blown experiment consisting only of components that are known to be suitable for integration with a lander instrument package. The remaining step was K analysis of the glass formed by quenching the flux+melt. Ken Farley demonstrated by ICPMS analysis that these glasses are homogeneous in chemical composition, and so any method capable of extracting and analyzing the isotopic composition of K will be suitable. The partner research program at JPL continued advancing the sensitivity and precision of its mass spectrometric measurements of K released by laser ablation of silicates.
Project Results
Project Inception
